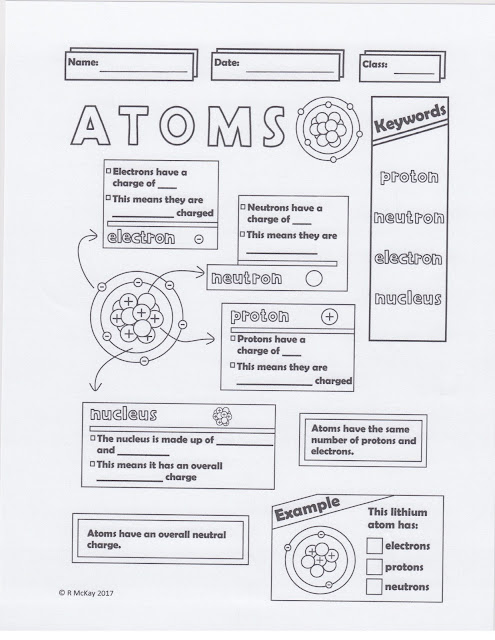
ESSENTIAL QUESTION: How do atoms combine?
LEARNING TARGET: Analyze the properties of substances to determine if they are mixtures or pure substances.
BENCHMARKS: SC.8.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-Analyze the properties of substances to determine if they are mixtures or pure substances.
-Differentiate among pure substances, mixtures, and solutions.
-Utilize the pH scale to measure acids and bases.
-Take the Unit 3 Assessment
- Data chat
BELL RINGER: Correct most missed top questions
VOCABULARY: pure substance, mixture, heterogeneous mixture, homogeneous mixture, solution, solvent, solute, colloid, colloidal suspension, dilute suspension, concentrated solution, solubility, saturated solution
HOME LEARNING: HL 4 Martian Periodic Table
AGENDA
WHOLE GROUP
Absent students took the Unit 3 assessment, while other students corrected their exams.
We then reviewed the top most missed questions.
Home learning 4, the Martian periodic table was explained. You can find a copy of the home learning above.
Students then worked independently on their lowest benchmarks.