ESSENTIAL QUESTION: Why and how has the atomic theory changed over time?
LEARNING TARGET: What makes up an atom?
BENCHMARKS: SC.8.P.8.6
LEARNING OBJECTIVES: Students will be able to:
-Describe how phase changes in substances are affected by temperature.
-Describe the parts of an atom.
BELL RINGER: FCAT Practice: SC.8.N.1.3 The Practice of Science
Before 1896, many scientists concluded that light could not pass through black paper. In
1896, Henri Becquerel observed that uranium salts could cause a plate covered by black
paper to react as if light had reached it.
How did this observation affect conclusions about light passing through black paper?
A. Scientists ignored Becquerel’s evidence because uranium is dangerous to use, so his
study was not valid.
B. Scientists repeated Becquerel’s experiment until it no longer worked and reported that
his conclusions were not valid.
C. Scientists had to revise their earlier conclusion because evidence from Becquerel did
not support the original conclusion.
D. Scientists stood behind the earlier conclusion that black paper blocks light because it
was already an accepted conclusion.
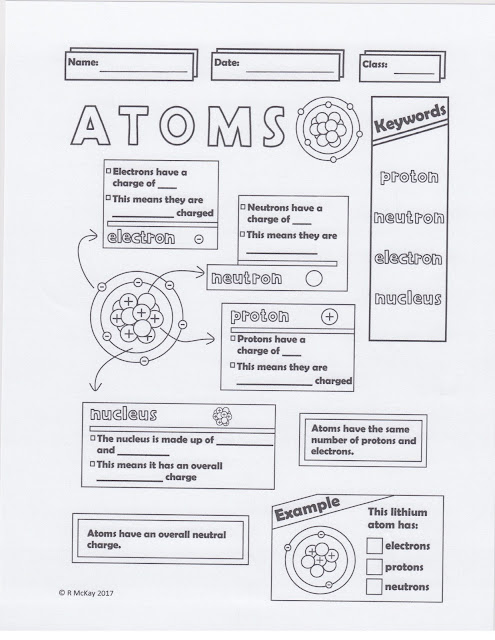
VOCABULARY: matter, chemistry, substance, element, atom, Atomic mass, periodic table, nucleus, proton, atomic number, neutron, electron, mass number
HOME LEARNING: HL 1 Atomic Structure
AGENDA
WHOLE GROUP
Students reviewed the Practice of Science by completing and discussing the bell ringer above.
Students watched a BrainPop on the Periodic Table and wrote a question from the video that other class members had to answer. You can watch the BrainPop video by visiting the site at: BrainPop Periodic Table. Once on the site, use the user name and password:
Username: amsbartow
Pasword: ams13
We then completed the notes/handout on atoms and their parts. You can find the handout below.
Home learning 1, which can be found above, was explained. It is due next class period.
Students also had data chats. Students had the opportunity to work on Edgenuity.